| |




- Analysing an active site
- Building Loops
- Building a functionnal unit from a monomer
- Crystal Symmetries
- Electron Density Maps
- Energy minimisation
- Fitting Residues into Electron Density
- Homology modelling
- Making Phi/Psi statistics
- Superposing Proteins





by N.Guex &
T.Schwede
|
|
Electron Density maps : fatty acid binding
protein
To complete this tutorial, you will need
to download some material (coordinates and electron density map of the lysozyme).
This data set has kindly been made available by Dr. Jim Thompson, Dr. Nate
Winter and Prof. Len Banaszak
- material for Macintosh (.hqx 855 Kb)
- material for PC (.zip 553 Kb)

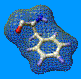
Stereo detail of a part of the fatty acid binding protein. Rendering
with POV-Ray.
Step by Step
- First of all, if you are using a Macintosh, make sure that Swiss-PdbViewer
has at least 6000Kb of free memory to run (click on the icon, press
Command I, and adjust the memory allocated to the program). Then launch
it.
- Open the pdb file lfabp .pdb.
- Then, verify that the checkbox "center upon loading" of the "General
preference" is not checked. If it was checked, uncheck it, close
the protein and reload it.
- Center the view by hitting the "=" key of the numerical keypad right
mouse button on the PC), and enable the "Slab" item of the Display Menu.
- Click on the first button at the top left of the main window and set
the "slab depth" to 8Å in order to limit the quantity of information
displayed on screen at the same time.
- Select the Open DN6 Map from the file menu and load the file "lfabp.dn6".
A dialog will appear. The upper part provides some information about
the unit cell size: the length along its axis, and the angle between
the axis. Note that the axis are not necessarily orthogonal. It all
depends on the crystal symmetry. In this case, there is an angle of
120° between axis x and y.

Below are some information on the number of sections available in
the map. As a whole edm is pretty heavy to handle, it is advised to
display only a subpart of it. In our case, we will display only from
sections 50, 20 and 40 (along X,Y and Z respectively) to sections
80, 50 and 56.
The last part of the dialog let you decide what will be the cutoff
density for the contouring, i.e. decide what are the limit
between a dense zone and a zone with low edm.
Type 2.5 in the field and accept these settings. Amino-acids belonging
to the antiparalell strand 99-113 are perfectly fitted within the
electron density map. What you see is in fact the final stage of the
process. During initial stages of the resolution of a protein, edm
are much more messy. Hold down the shift key while clicking and moving
the mouse toward you to move the slab toward you. Some new parts of
the protein will be revealed, all of which are not contoured. Remember
that only a subpart of the edm was selected to be displayed. Play
a little with the protein; look at the anti parallel b-stands, and
so on.
- Now go to the control Panel and click on the Asp106 while holding
down the control key (shift Control for PC). This will automatically
center its alpha carbon in the view. Zoom in. Note that the sidechain
is not buried into the edm. It means that the location of the carboxyl
group is not very well defined in space. As a matter of fact, sidechains
of surface residues tend to be more flexible and therefore do not diffract
very well.
- Go back to the edm preferences dialog, and enable the second contour
with a 1.5 sigma value. Click on the dotted checkbox, and accept the
settings. A second contour, englobing more parts of the structure, among
those the CG of Asp106 appears with an other colour. By decreasing the
sigma value, you contour parts of the map that have a smaller electron
density than when you use a high value.
- Go back to the same dialog by hitting command+Y (control+Y for the
PC), disable the display of the second contour and enable the dotted
checkbox of the first contour. The display will now look like a cloud
of dots, and the amino-acids appear more clearly within the density
(this mode will be useful until I add a Z-buffer and also maybe a color-distance
fading feature).
As you have noticed, only the part of the edm you have selected to be
displayed are actually shown. If you want to inspect all amino-acids
one by one to see how well they fit into the edm, it would be useful
to display only subpart of the edm corresponding to the residue you
currently closely inspecting. Well, this is possible.
- Bring back the edm dialog and enable the radio button entitled "Display
around CB". By default, only parts of the edm lying within 5Å
of the carbon beta of the currently centred residue (Ion our case Asp106)
will be displayed in each direction along the unit cell axis.
- Center the view on the next residue (simply hit the right arrow key)
and a new portion of the edm will be displayed. Navigate down along
the peptide to look at the edm, and stop on the residue Asp87. Here
again, you can see that only no density is displayed around the sidechain.
It is therefore very useful to be able to change the sigma value used
for the contouring. This can be done without using the edm preference
dialog: simply hit the down arrow key and the sigma contouring value
will be decreased by 0.1. Hit the down arrow key 10 times more and look
how the edm displayed increasingly covers the atoms. (note: typing the
down arrow key while maintaining the shift key down will decrease the
sigma value for the second contouring value).
- Colour the protein by B-factor. Look at how the sidechain of the Asp87
appears reddish. This means indeed that the electron density is badly
defined in this area.
- Now Bring back the edm dialog and enable the coarse contouring along
the Z axis. The contouring will be less fine bet the display should
appear slightly less cluttered and faster.Play a little with the coarse
checkboxes to look at the effect. Usually, working with one or two axis
coarsely contoured allows a good rendering speed without noticeably
affecting the display precision.
- Click on the small text icon located at the right of the earth icon.
in the main window. The text file will the coordinates of the currently
active pdb file are displayed. Scroll down to the residue Asp87, and
look at the B-factor of atoms OD1 and OD2 (last column containing digits).
It is 85.39 and 84.53, which is very high. Colouring a protein by B-factor
allows to immediately identify regions that are more accurately fixed
in space than other (usually the core of the protein).
Go to next tutorial
|
 ExPASy Home page
ExPASy Home page ExPASy Home page
ExPASy Home page





 ExPASy Home page
ExPASy Home page