Helix-Sheet Packing
Because the periodicity of an alpha-helix is different to that of a
beta-sheet, a regular intercalated interface, such as that proposed between
a pair of alpha-helices (refer to helix-helix packing),
would not seem to be possible.
Earlier studies showed a discrepancy as to whether there is regular
local
intercalation between a single beta-sheet residue and its four surrounding
helix residues i+1, i+4, i+5, i+8 as in Figs. 1 and 2 (Cohen et
al., 1982) or no regular intercalation at all (Janin and Chothia, 1980).
The latter point of view is of a largely flat surface with a few irregular
intercalations.
The Complementary Twist Model
(Refer to Chothia, 1984)
The Interface Residues on the Alpha-Helix
The helix residues which pack against the beta-sheet tend to belong to
two neighbouring ridges of the ±4n form (see previous section on
helix-helix
packing). This is illustrated in the diagrams (Figs. 1 and 2) below.
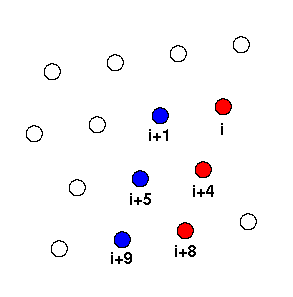
Fig. 1. The cylindrical surface of an alpha-helix unrolled and flattened;
each circle represents a side chain. The N-terminal end is at the top.
Two neigbouring ±4n ridges are highlighted in red and blue. After
Chothia (1984)
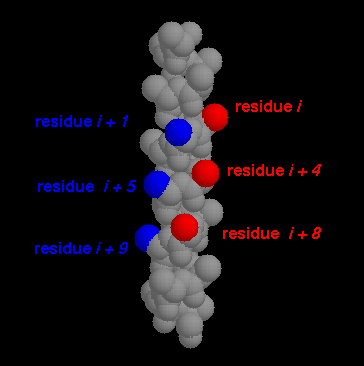
Fig. 2. The same grooves highlighted in an alpha-helix.
 To view these highlighted
residues in an alpha helix, download this
model
helix(19Kb), then apply this
RasMol script
To view these highlighted
residues in an alpha helix, download this
model
helix(19Kb), then apply this
RasMol script
Only three residues in each ridge are indicated, because this is approximately
the extent of the interface with a typical beta-sheet. This is due to the
twist of the sheet: the strands twist away from the helix residues situated
further along the grooves.
The Interface Residues on the Beta-Sheet
The typical interface between an alpha-helix and a beta sheet involves
three neighbouring strands of the sheet. This is indicated in Fig. 3 below.
The central strand has three residues in contact with the helix, while
the outer strands twist away from the helix in one direction. Thus, due
to the overall twist of the beta sheet, there is a 'plateau' of residues
diagonally spanning two corners.
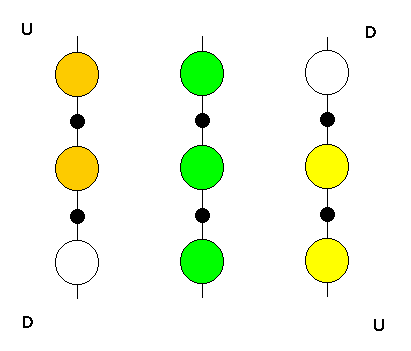
Fig. 3 Three neighbouring strands of a beta-sheet forming an interface
with an alpha-helix. The small black circles represent the side chains
of residues on the lower surface, while the large circles are side chains
on the upper surface. The shaded circles are those residues which, due
to the twist of the sheet, form a raised surface extending from one upper
(U) corner to the other; the remaining two residues are in a down
(D) position. After Chothia (1984)
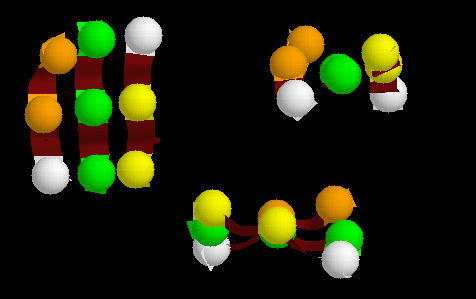
Fig. 4 The same residues in a beta-sheet (the positions highlighted
are of alpha carbons). Three orthogonal views are shown, indicating the
raised residues, resulting from the twist. This sheet is in the PDB structure
1ofv (flavodoxin; Luschinsky et al., 1991).
 This may be viewed using
RasMol by downloading the flavodoxin PDB file:
1ofv
(86Kb) [Bbk|BNL|ExP|Waw|Hal]
and then using this script to highlight beta-strands
1, 2 and 3. Then keep this window open (see below).
This may be viewed using
RasMol by downloading the flavodoxin PDB file:
1ofv
(86Kb) [Bbk|BNL|ExP|Waw|Hal]
and then using this script to highlight beta-strands
1, 2 and 3. Then keep this window open (see below).
The Complementary Twist in a Real Protein
We will now examine a helix-sheet interface in the flavodoxin structure
(1ofv) that you have just looked at (see above).
 Three scripts are provided:
Three scripts are provided:
-
script to show the same sheet with the entire sidechains
of the upper-surface residues. Notice how the sidechain conformations
comprise a flat surface. Residue 53 is a glycine; the alpha carbon is shown
as a spacefilling atom.
-
script to show the helix (helix 1) which packs
against the three strands of sheet which you have previously viewed.
-
script to show the helix and sheet. Which alpha-helix
side chains are involved in the interface in this case? Are any other ±4n
ridges involved? Try illustrating this with RasMol.
Neighbouring alpha helices packing against beta-sheets
Consider two helices packing against each other and a beta-sheet. The typical
twist of a beta-sheet, which means that the angle between neighbouring
strands approximately 4.5Å apart is -19°, means that the two
helix axes could not be parallel. The twist of the sheet implies that the
expected angle between the two axes is about -40°. In the ridges-and-grooves
model, the closest helix-helix angle to this value is the 52° between
two helices with intercalating ±4n ridges. 80% of alpha-helices
which pack onto neighbouring helices and a beta-sheet were found
to exhibit this particular ridge-and-groove interaction with each other
(Chothia, 1984). Note that when a ±4n ridge fits into the
±3n grooves of a neighbouring helix, the expected angle between
the axes is 23° (refer to the page on helix-helix
packing).
Last updated 7th April '97



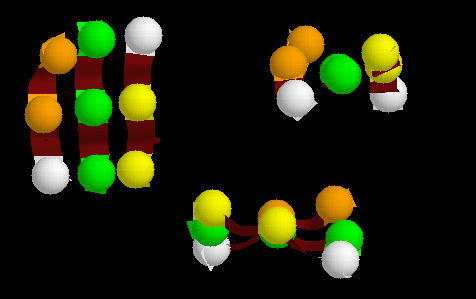
![]() Three scripts are provided:
Three scripts are provided: