 |
|
|
Helix-Helix Packing
When two alpha-helices pack against each other, the side-chains at the interface are buried. The two interface areas have complementary surfaces. The surface of an alpha-helix can be thought of as consisting of grooves and ridges, like a screw thread : for instance, the side chains of every 4th residue form a ridge (because there are 3.6 residues per turn). The direction of this ridge is 26° from the direction of the helix axis. Therefore if 2 helices pack such that such a ridge from each fits into the other's groove, the expected angle between the two is 52°. In fact, in the distribution of this angle between packed alpha-helices, there is a peak at 50°. Besides the type of ridge described (termed a ±4n ridge), ridges can be formed by other staggerings of residues, such as every 3rd residue (forming a ±3n ridge), or indeed every residue. Which ridges are involved in packing depends on the size and conformations of the side chains at these relative positions. The ±4n ridge is believed to be the most common because residues at every 4th position have side-chains which are more closely aligned than in ±3n or ±n ridges as indicated below.
The interaxial distance between packed helices varies from 6.8-12.0Å, the mean being 9.4 Å;the mean interpenetration of atoms at the interface is 2.3Å. Therefore it is mainly side chains which make the contacts between the helices.
Click here for a diagram (9Kb) indicating the relative angles of the ±4n and ±3n ridges.
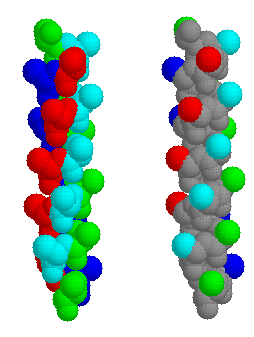
Fig. 1 ±4n ridges in a model alpha helix. All the residues are Alanines. On the left, the whole of each residue is coloured, to indicate the ridge orientation. On the right, only the beta carbon atoms are highlighted: residues 2,6,10,14,18 and 22 are green; 3,7,11,15,19 cyan; 4,8,12,16,20 red; 5,9,13,17,21 blue. The second representation better illustrates the ridges between the grooves.
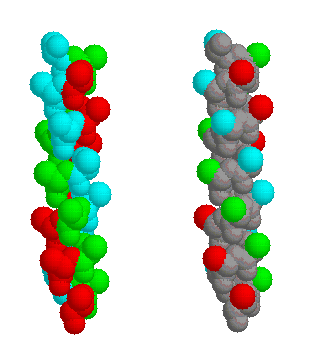
Fig. 2 ±3n ridges in the same model alpha helix. The ridges are coloured red (residues 4,7,10,13,16,19,22), cyan (2,5,8,11,14,17,20) and green (3,6,9,12,15,18,21); on the left the whole residue is highlighted, on the right only the side chains.
Of course, there is only one ±n ridge, which includes all the helix residues. Perhaps a good way of indicating this ridge in the model helix is to use the RasMol commands select all followed by colour group.
Note that ±2n ridges are not relevant in alpha helices, because 'neigbouring' residues are on opposite sides of the helix, so that this effectively describes two ±4n ridges.
We will now examine the intercalation between the ±4n ridges and grooves of the B-helix and G-helix. These helices are composed of residues 21-35 and 102-118, respectively.
Apply the following scripts to the structure, to highlight the helices:
The intercalating sidechains at this interface are mostly hydrophobic: Phe 28 and Leu 32 (red), Val 108 and Phe 112 (orange), Met 111 and Phe 115 (white) and Ala 31 and Lys 35 (cyan). Note that most of the surface of the latter is nonpolar, while the charged NZ atom points towards the solvent, rather than the G-helix.
The intercalation may appear more clearly if you use the RasMol slab mode; use this script.
Colouring the helices uniformly may also help; cut and paste the following into your RasMol command line window:
![]() Now download the structure
of sperm whale myoglobin (Phillips and Schoenborn, 1981)
1mbd
(147Kb) [Bbk|BNL|ExP|Waw|Hal]
Now download the structure
of sperm whale myoglobin (Phillips and Schoenborn, 1981)
1mbd
(147Kb) [Bbk|BNL|ExP|Waw|Hal]
Helices B and E pack together approximately at 90°. This is made possible by 'notches' in an ±4n ridge on each, due to the absence of a side chain (i.e. the presence of a glycine residue) in each helix.
A different view is obtained with another script; or look at the image (20Kb).
 |
|
|
Last updated 7th April '97