Super Secondary Structure
PART III
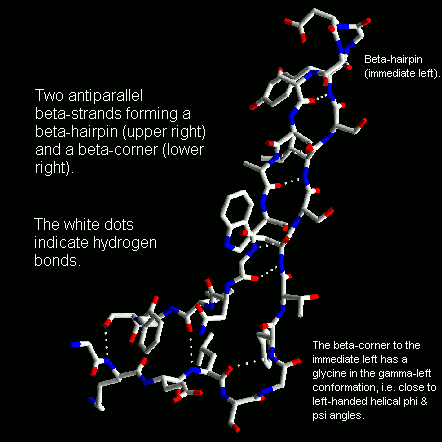
Beta-corners
Beta-strands have a slight right-handed twist such that when they pack
side-by-side to form a beta-sheet, the sheet has an overall left-handed
curvature. Antiparallel beta-strands forming a beta-hairpin can accommodate
a 90 degree change in direction known as a beta-corner. The strand on the
inside of the bend often has a glycine at this position while the other
strand can have a beta-bulge. The latter involves a single residue in the
right-handed alpha-helical conformation which breaks the hydrogen bonding
pattern of the beta-sheet. This residue can also be in the left-handed
helical or bridging regions of the Ramachandran plot. Take a look at this
example
structure file from penicillopepsin (3APP) to examine with RasMol.
Beta-corners are observed to have a right-handed twist when viewed from
the concave side.
Helix hairpins
A helix hairpin or alphaalpha-hairpin refers to the loop connecting two
antiparallel alpha-helical segments. Clearly, the longer the length of
the loop the greater the number of possible conformations. However, for
short connections there are a limited number of conformations and for the
shortest loops of two or three residues, there is only one allowed conformation.
Antiparallel alpha-helices will interact generally by hydrophobic interactions
between side chains at the interface. Therefore, hydrophobic amino acids
have to be appropriately positioned in the amino acid sequence (one per
turn of each helix) to generate a hydrophobic core. Efimov has analysed
the conformations of alpha-alpha-hairpins and some of his results are summarised
below.
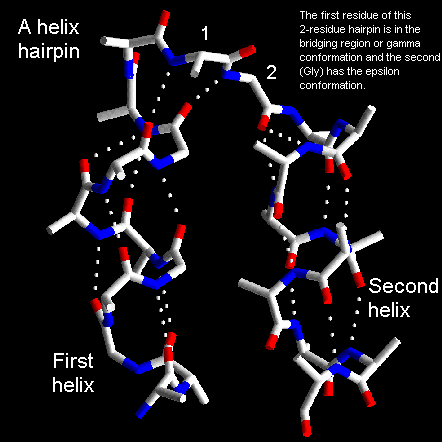
The shortest alpha-helical connections involve two residues which are
oriented approximately perpendicular to the axes of the helices. Analysis
of known structures reveals that the first of these two residues adopts
phi and psi angles in the bridging or alpha-helical regions of the Ramachandran
plot. The second residue is always glycine and is in a region of the Ramachandran
plot with positive phi which is not available to other amino acids. Here
is an example structure file to look at with
RasMol.
Three residue loops are also observed to have conformational preferences.
The first residue occupies the bridging region of the Ramachandran plot,
the second adopts the left-handed helical conformation and the last residue
is in a beta-strand conformation.
Four-residue loops adopt one of two possible conformations. One is similar
to the three residue loop conformation described above except that there
is an additional residue in the beta-strand conformation at the fourth
position. The other conformation involves the four residues adopting bridging,
beta, bridging, and beta conformations, respectively.
j.cooper 26/1/95
Last updated 7th April '97