Super Secondary Structure
PART I
Introduction
Secondary structure elements are observed to combine in specific geometric
arrrangements known as motifs or supersecondary structures. In this section
we will look at motifs consisting of no more than three secondary structure
elements. Larger motifs such as the greek key will be examined in the sections
on tertiary structure and protein folds.
Beta-hairpins
Beta-hairpins are one of the simplest supersecondary structures and are
widespread in globular proteins. They occur as the short loop regions between
antiparallel hydrogen bonded beta-strands. In general a reverse turn (or
beta-turn, as it they are sometimes called) is any region of a protein
where there is a hydrogen bond involving the carbonyl of residue i and
the NH group of residue i+3. An alternative definition states that the
alpha-carbons of residues i and i+3 must be within 7.0 Angstroms. The structures
of reverse turns are outlined in the section on peptide geometry. In this
section we will concentrate on those turns which occur between consecutive
beta-strands, known as beta-hairpins. Sibanda and Thornton have devised
a system for classifying beta-hairpins which is based on two conventions
for defining loop regions. In this section we will not go into such details
as the objective is indicate the most commonly observed hairpin loop structures.
Beta-hairpin loops adopt specific conformations which depend on their
lengths and sequences. Sibanda and Thornton have shown that 70% of beta-hairpins
are less than 7 residues in length with the two-residue turns forming the
most noticeable component. These two-residue beta-hairpins all adopt one
of the classical reverse turn conformations with an obvious preference
for types I' and II'. Type I 2-residue hairpins also occur but with lower
abundance. This contrasts with reverse turns where types I and II tend
to dominate. In beta-hairpins the type I' turn has the correct twist to
match the twist of the beta-sheet and modelling studies indicated that
if either type I or type II turns were to connect the antiparallel beta-strands,
they would diverge within a short distance from the turn.
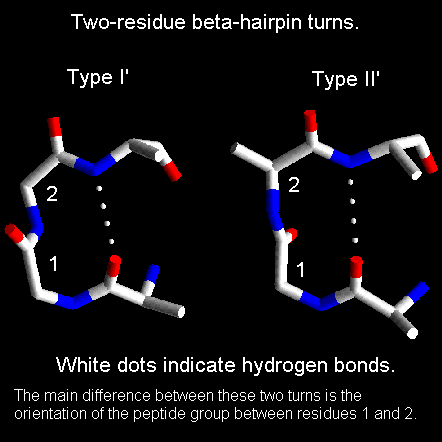
Two-residue beta-hairpins
-
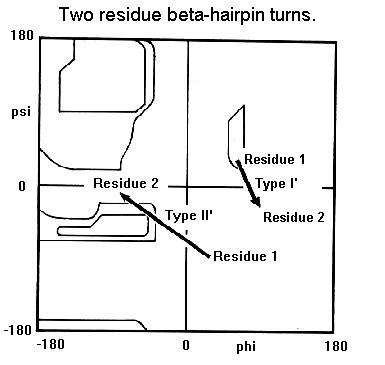
Type I'.The first residue in this turn adopts the left-handed alpha-helical
conformation and therefore shows preference for glycine, asparagine or
aspartate. These residues can adopt conformations with positive phi angles
due to the absence of a side chain with glycine and because of hydrogen
bonds between the side chain and main chain in the case of asparagine or
aspartate. The second residue of a type I' turn is nearly always glycine
as the required phi and psi angles are well outside the allowed regions
of the Ramachandran plot for amino acids with side chains. Were another
type of amino acid to occur here there would be steric hindrance between
its side chain and the carbonyl oxygen of the preceding residue.
-
Type II'. The first residue of these turns has a conformation which
can only be adopted by glycine (see below Ramachandran plot). The second
residue shows a preference for polar amino acids such as serine and threonine.
-
Type I. Both residues of these turns adopt alpha-helical conformations.
j.cooper 26/1/95
Last updated 7th April '97