[Next]
[Previous] [Up] [Top]
3.2 Sheets
3.2.1 Antiparallel
A residue in an antiparallel beta strand has values of -139 and +135 degrees
for the backbone dihedral angles phi and psi, respectively. Antiparallel
beta sheets are thought to be intrinsically more stable than parallel sheets
due to the more optimal orientation of the interstrand hydrogen bonds and
that peptide bond dipoles of nearest neighbors within an strand cancel
whereas in the parallel sheet, components of the dipoles parallel to the
strands align and may interact unfavorably. However, in their exhaustive
survey of hydrogen bonds in protein three-dimensional structures, Baker
& Hubbard (1984) found no significant difference in the linearity of
the hydrogen bonds in parallel and antiparallel sheets. An example of a
mixed beta sheet containing an antiparallel component is given using the
protein thioredoxin (Figure 10 ).
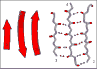
Figure 10. The three-stranded antiparallel beta
sheet in the protein thioredoxin (2TRX.PDB). The three antiparallel strands
are shown in both cartoon format (left) and in stick form containing backbone
atoms N, CA, C, and O' (right). Hydrogen bonds are identified by arrows
connecting the donor nitrogen and acceptor oxygens. Strands are numbered
according to their relative position in the polypeptide sequence.
download 2TRX.PDB
download RasMol script for antiparallel
sheet
No Title - 31 MAY 96
written by Kurt D. Berndt
[Next] [Previous]
[Up] [Top]
Section 8 Index
Generated with CERN WebMaker