AMINO ACID SIDE CHAINS. PART IV
in Four Easy Parts
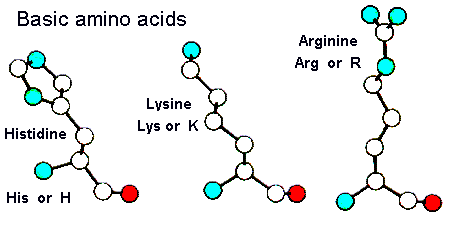
Basic amino acids
Of the basic amino acid side chains, histidine has the lowest pKa (around
6) and is therefore neutral at around physiological pH. This amino acid
occurs very frequently in enzyme active sites as it can function as a very
efficient general acid-base catalyst. It also acts as a metal ion ligand
in numerous protein families. Lysine and arginine are more strongly basic
and are positively charged at physiological pH's. They are generally solvated
but do occasionally occur in the interior of a protein where they are usually
involved in electrostatic interactions with negatively charged groups such
as Asp or Glu. Lys and Arg have important roles in anion-binding proteins
as they can interact electrostatically with the ligand. Here is a
pdb-file
for RasMol.
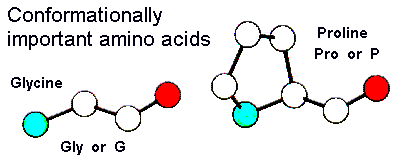
Conformationally important residues
Glycine and proline are unique amino acids in that they appear to influence
the conformation of the polypeptide. Glycine essentially lacks a side chain
and therefore can adopt conformations which are sterically forbidden for
other amino acids. This confers a high degree of local flexibility on the
polypeptide. Accordingly, glycine residues are frequently found in turn
regions of proteins where the backbone has to make a sharp turn. Glycine
occurs abundantly in certain fibrous proteins due to its flexibility and
because its small size allows adjacent polypeptide chains to pack together
closely. In contrast, proline is the most rigid of the twenty naturally
occurring amino acids since its side chain is covalently linked with the
main chain nitrogen. Here is a pdb-file for RasMol.
Last updated 9h Oct'96