Super Secondary Structure
PART II
Beta-hairpins continued
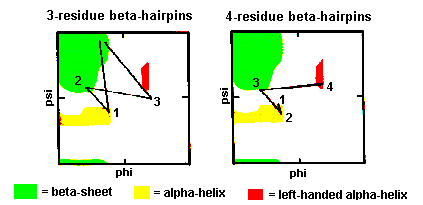
Three-residue beta-hairpins
Normally the residues at the ends of the two beta-strands only make one
hydrogen bond as shown below. The intervening three residues have distinct
conformational preferences as shown in the Ramachandran plot. The first
residue adopts the right-handed alpha-helical conformation and the second
amino acid lies in the bridging region between between alpha-helix and
beta-sheet. Glycine, asparagine or aspartate are frequently found at the
last residue position as this adopts phi and psi angles close to the left-handed
helical conformation. For RasMol-MIME, here is a pdb-file

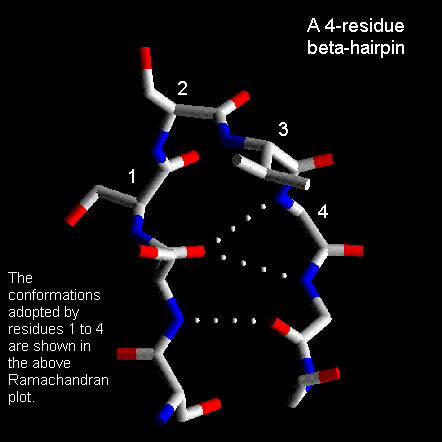
Four-residue beta-hairpins
These are also quite common with the first two residues adopting the alpha-helical
conformation. The third residue has phi and psi angles which lie in the
bridging region between between alpha-helix and beta-sheet and the final
residue adopts the left-handed alpha-helical conformation and is therefore
usually Gly, Asp or Asn. Here is a coordinate structure
file to view in RasMol.
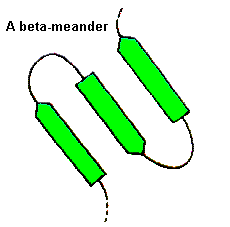
Longer loops
For these, a wide range of conformations is observed and the general term
'random coil' is sometimes used. Consecutive antiparallel beta-strands
when linked by hairpins form a supersecondary structure known as the beta-meander.
j.cooper 26/1/95
Last updated 7th April '97






