Potential
energy curve for the N-C-O Bond Angle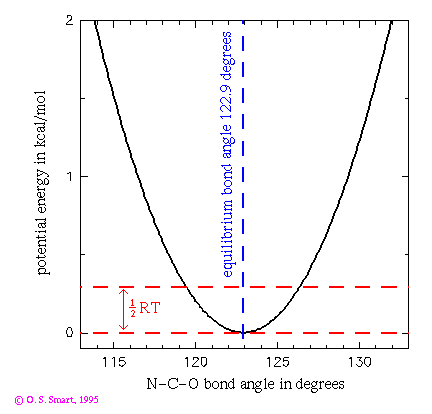
This graph shows the potential energy for a N-C-O bond angle using a
harmonic potential with the parameters given in the
AMBER
potential energy function. Such a term would partly control the angular
vibrations of the carbonyl oxygen on the main chain of a polypeptide chain.
The bond angle constant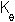 is 80 kcal/(mol.degrees^2) and the equilibrium bond angle (
is 80 kcal/(mol.degrees^2) and the equilibrium bond angle (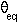 )
is 122.9 degrees. The dashed line indicates an energy of 0.29 kcal/mol
which is equal to 1/2RT at a temperature of 300K. This is the energy that
an individual degree of freedom can expect at this temperature and indicates
that a this bond could be expect to be undergoing vibrations of the order
of 4 degrees at room temperature. If we assumes that the N and C atoms
remain mostly static then this would entail motion in this degree of freedom
of the order of 0.09 Å for the oxygen atom (as the C-O bond is 1.229
Å long). It is interesting to compare this to a motion of approximately
0.03 Å amplitude to vibrations of the C=O bond (link
to graph of this motion). (This is not quite the whole story as there
would be another bond angle term for the atoms C_alpha-C-O also restricting
the motion).
)
is 122.9 degrees. The dashed line indicates an energy of 0.29 kcal/mol
which is equal to 1/2RT at a temperature of 300K. This is the energy that
an individual degree of freedom can expect at this temperature and indicates
that a this bond could be expect to be undergoing vibrations of the order
of 4 degrees at room temperature. If we assumes that the N and C atoms
remain mostly static then this would entail motion in this degree of freedom
of the order of 0.09 Å for the oxygen atom (as the C-O bond is 1.229
Å long). It is interesting to compare this to a motion of approximately
0.03 Å amplitude to vibrations of the C=O bond (link
to graph of this motion). (This is not quite the whole story as there
would be another bond angle term for the atoms C_alpha-C-O also restricting
the motion).
Oliver Smart
© O.S.
Smart 1995, all rights reserved
Back to looking at bond angle
interaction
Back to main Molecular Forces index
Back to main PPS course Index
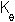 is 80 kcal/(mol.degrees^2) and the equilibrium bond angle (
is 80 kcal/(mol.degrees^2) and the equilibrium bond angle (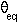 )
is 122.9 degrees. The dashed line indicates an energy of 0.29 kcal/mol
which is equal to 1/2RT at a temperature of 300K. This is the energy that
an individual degree of freedom can expect at this temperature and indicates
that a this bond could be expect to be undergoing vibrations of the order
of 4 degrees at room temperature. If we assumes that the N and C atoms
remain mostly static then this would entail motion in this degree of freedom
of the order of 0.09 Å for the oxygen atom (as the C-O bond is 1.229
Å long). It is interesting to compare this to a motion of approximately
0.03 Å amplitude to vibrations of the C=O bond (link
to graph of this motion). (This is not quite the whole story as there
would be another bond angle term for the atoms C_alpha-C-O also restricting
the motion).
)
is 122.9 degrees. The dashed line indicates an energy of 0.29 kcal/mol
which is equal to 1/2RT at a temperature of 300K. This is the energy that
an individual degree of freedom can expect at this temperature and indicates
that a this bond could be expect to be undergoing vibrations of the order
of 4 degrees at room temperature. If we assumes that the N and C atoms
remain mostly static then this would entail motion in this degree of freedom
of the order of 0.09 Å for the oxygen atom (as the C-O bond is 1.229
Å long). It is interesting to compare this to a motion of approximately
0.03 Å amplitude to vibrations of the C=O bond (link
to graph of this motion). (This is not quite the whole story as there
would be another bond angle term for the atoms C_alpha-C-O also restricting
the motion).
