 Allostery: Haemoglobin (part 2)- Structure
Allostery: Haemoglobin (part 2)- Structure![]() Index
to Course Material
Index
to Course Material![]() Index
to Section 12
Index
to Section 12![]() Part
1;
Part
1; ![]() Part
3
Part
3
 Click
here for a diagram of haem.
Click
here for a diagram of haem.![]() Apply this SCRIPT
to the 1hho haemoglobin structure (see above).
Apply this SCRIPT
to the 1hho haemoglobin structure (see above).
The haem group is situated between helices E and F, and is surrounded by non-polar residues except for the its carboxylate groups exposed at the protein surface (here is a diagram, 82Kb), and for two of the protein's histidine side chains (here is a SCRIPT for the 1hho structure, which highlights the haem and colours these histidines blue). One of these (His 87 in alpha subunit, His 92 in beta), part of helix F, binds directly to the iron atom of the haem group (the NE2 atom of the His side chain occupies one of the six coordination positions of the iron). This His is in each subunit called the proximal histidine. The distal histidine occurs in helix E (His 58 in alpha subunit, His 63 in beta). This is near to the opposite coordination position, but does not occupy it; this coordination site is occupied by oxygen in oxyhaemoglobin.
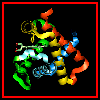 Here are orthogonal views of (oxy)haemoglobin illustrating the positions
of the proximal and distal histidines in relation to the haem.
Here are orthogonal views of (oxy)haemoglobin illustrating the positions
of the proximal and distal histidines in relation to the haem. ![]() SCRIPT
for 1hho
SCRIPT
for 1hho
The above script labels each of the helices. Note that the alpha subunit has only 7 helices (helix D is missing, or described as 'degenerate' consisting of only 2 residues), but they are named as in the scheme for the typical 8-helix globin fold.
Is helix D present in the beta subunit? Of how many residues does it consist? Refer to the heading of the pdb file.
 The
orientation of the four subunits in the tetrameric haemoglobin molecule
is indicated in this diagram.
The
orientation of the four subunits in the tetrameric haemoglobin molecule
is indicated in this diagram.
![]() The crystal structure of deoxyhaemoglobin
is a complete tetramer (2 alpha and 2 beta subunits) 2hhb (419Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT provides a similar rendition to
the above diagram. Keep the RasMol window open with the 2hhb
structure.
The crystal structure of deoxyhaemoglobin
is a complete tetramer (2 alpha and 2 beta subunits) 2hhb (419Kb)
[Bbk|BNL|ExP|Waw|Hal]
This SCRIPT provides a similar rendition to
the above diagram. Keep the RasMol window open with the 2hhb
structure.
Swiss-3D prot provide this diagram.
What type of symmetry does the tetramer have? For further information refer to the symmetry page of the previous section (on Quaternary Structure).
 This diagram
illustrates (1) and (2) of the above.
This diagram
illustrates (1) and (2) of the above.  This
diagram illustrates (3) and (4).
This
diagram illustrates (3) and (4). ![]() SCRIPT
SCRIPT
This SCRIPT displays only the backbone atoms, except for the residues involved in the interactions above, whose side chains are displayed. They are also labelled; to turn these off type:
select label offThe haem group of deoxyhaemoglobin is domed rather than planar. This relates to the ionic radius of the iron, which is in a high-spin Fe(II) state. The iron is too large (radius 2.06Å) to fit in the ring of nitrogens with which it coordinates; it is 0.6Å out of the plane of the ring, which is therefore distorted.

 Close-up
Close-up
 and a perpendicular view
and a perpendicular view
![]() SCRIPT To obtain
the perpendicular view, type:
SCRIPT To obtain
the perpendicular view, type:
rotate y -90 zoom 450 slab
In contrast to deoxyhaemoglobin, the haem group is planar and the iron ion lies in the plane of the ring, as it is in a low-spin Fe(II) state with a smaller radius (1.98Å). All six coordination positions of the ion are occupied: the bound oxygen molecule accounts for the sixth.
Perpendicular view:
This rendition is obtained by applying the SCRIPT used earlier in this page to the 1hho oxyhaemoglobin structure (or the tetrameric form of 1hho; see below). The link to the 1hho crystal structure is at the top of this page.
Recall that the 1hho oxyhaemoglobin crystal structure contains only one alpha and one beta subunit (because the other half of the tetramer is related by crystal symmetry). You can also download the complete oxyhaemoglobin tetramer (333Kb) (provided by Brookhaven).
Last updated 8th Jul '96