Multienzyme Complexes
In a number of metabolic pathways, several enzymes which catalyze different
stages of the process have been found to be associated noncovalently, giving
a multienzyme complex. The proximity of the different types of enzymes
increases the efficiency of the pathway: the overall reaction rate is increased
with respect to catalysis by unassociated units, and side reactions are
minimized. In some cases molecular mechanisms have been identified for
the transfer of metabolites from one enzyme to the next within the complex.
Later in the course we will be studying enzymes in general.
Pyruvate Dehydrogenase Complex
This multienzyme complex catalyses the conversion of pyruvate and
coenzyme
A (CoA) to acetyl CoA.
The reaction
There are four stages in this pathway, which are catalyzed by three enzymes:
-
"E1" - pyruvate dehydrogenase
-
This enzyme catalyzes the decarboxylation of pyruvate. This involves the
prosthetic group thiamine pyrophosphate, or TPP.
-
"E2" - dihydrolipoyl transacetylase
-
Two steps of the pathway are catalyzed by this enzyme:
-
oxidation of the 2-carbon (acetyl) unit, which is transferred to the lipoamide
prosthetic group of the enzyme, giving an acetyllipoamide group
-
transfer of the acetyl group from the lipoamide to CoA, giving acetyl CoA
-
"E3" - dihydrolipoyl dehydrogenase
-
Finally, this enzyme regenerates the oxidized form of lipoamide. This involves
the FAD prosthetic group.
Note that TPP, lipoamide and FAD are catalytic cofactors which remain
unaltered by the net reaction, whereas CoA and NAD+ are stoichiometric
cofactors; the overall reaction is:
pyruvate + CoA + NAD+ ----> acetyl CoA + carbon dioxide + NADH
The four stages are summarized in this diagram
.
Note that the lipoamide cofactor of E2 reacts with the product
(hydroxyethyl-TPP) from E1, and (in its modified form,
dihydrolipoamide,
formed by the third step) interacts with E3. It is for this reason
that the interaction rate is increased by proximity of all three enzymes,
and in fact the lipoamide group is a long flexible arm about 14Å
long. It is covalently bonded to a specific lysine
residue of the enzyme. The movement of the arm may be driven by a change
in the net charge of the lipoyl group, depending on the ionization of the
sulphydryl groups.
The structure of the complex
In isolation, E2 forms a homo-24-mer, believed to have cubic symmetry.
The E1 and E3 subunits each form homodimers. Electron micrographs
indicate that the whole complex has a cubic arrangment, in which an E3
dimer associates with each face of the E2 24-mer cube, while an
E1
dimer is positioned on each edge of the cube.
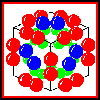 Click here for a diagram of this model.
Click here for a diagram of this model.
Refer to Reed(1974).
The Electron Transport Chain
Oxidative phosphorylation occurs in the mitochondria. The various
components of the electron transport chain are situated in the inner membrane
of this organelle. Oxidation of NADH and FADH2 (produced by the citric
acid cycle) results in free energy which is coupled to the phosphorylation
of ADP to form ATP (by ATP synthase, another complex in the inner mitochondrial
membrane). Electrons pass from lower to higher standard reduction potentials
in the chain in a series of redox reactions; the ultimate electron acceptor
is oxygen (O2).
The electron transport chain in fact consists of four multienzyme complexes.
These complexes are free to diffuse laterally through the membrane, and
are not present in the same numbers.
-
Complex I: NADH - Coenzyme Q reductase, 26 subunits, 850 kD. This
large protein includes one molecule of the prosthetic group flavin mononucleotide
, or FMN, and six to seven iron-sulphur clusters
-
Complex II: succinate - Coenzyme Q reductase, 5 subunits, 127 kD.
Includes:
-
FAD, covalently bound
-
three iron-sulphur clusters
-
cytochrome b560
-
Complex III: Coenzyme Q - cytochrome c reductase, 10 subunits,
280kD
-
 cytochrome b562 256b
(two molecules in asymmetric unit) (174Kb) [Bbk|BNL|ExP|Waw|Hal]
SCRIPT
cytochrome b562 256b
(two molecules in asymmetric unit) (174Kb) [Bbk|BNL|ExP|Waw|Hal]
SCRIPT
-
cytochrome b566
-
one iron-sulphur cluster
-
cytochrome c1
-
Complex IV: cytochrome c oxidase 7-8 subunits, 160-170kD
-
cytochrome a
-
cytochrome a3
Other components of the chain are succinate (between complexes I and II
in the sequence), Coenzyme Q (between II and III) and cytochrome
c
1ccr (90Kb) [Bbk|BNL|ExP|Waw|Hal]
SCRIPT
(between III and IV).
cytochrome
c
1ccr (90Kb) [Bbk|BNL|ExP|Waw|Hal]
SCRIPT
(between III and IV).
Electron micrographs have indicated the overall shape of Complex IV
and its orientation relative to the inner mitochondrial membrane. Techniques
involving chemical cross-linking and antibody-binding to different subunits
have led to a model of the whole complex, shown in this diagram
(after
Brunori and Wilson, 1982).The cytochrome c oxidase complex catalyzes
the oxidation of four reduced cytochrome c molecules; the binding
of cytochrome c to the complex mainly involves subunit II as indicated.
Refer also to
Simon
Brocklehurst's Multienzyme Complexes page.
References
Pyruvate Dehydrogenase Complex
-
Reed, L.J., (1974) Multienzyme complexes, Acc. Chem. Res. 7,
40-46
-
Stryer, L., (1981) Biochemistry, W.H. Freeman & Co., New York pp. 290-294
-
Voet, D. and Voet, J.G. (1990) Biochemistry, John Wiley & Sons, New
York, pp. 186-188
Electron Transport Chain
-
Brunori, M. and Wilson, M.T. (1982) Cytochrome oxidase, Trends Biochem.
Sci. 7, 295-299
-
Capaldi, R.A. (1982) Arrangement of proteins in the mitochondrial inner
membrane, Biochim. Biophys. Acta 695, 291-306
-
Capaldi, R.A., Malatesta, F. and Darley-Usmar, V.M. (1983) Structure of
cytochrome c oxidase, Biochim. Biophys. Acta 726,
135-148
-
Darnell, J., Lodish, H. and Baltimore, D. (1986) Molecular Cell Biology,
W.H. Freeman & Co., New York pp. 873-889
-
Poulos, T.L. and Kraut, J. (1980) A hypothetical model of the cytochrome
c peroxidase . cytochrome c electron transfer complex, J.
Biol. Chem. 255 10322-10330
-
Scott, R.A. (1989) X-ray absorption spectroscopic investigations of cytochrome
c oxidase structure and function, Annu. Rev. Biophys. Biophys.
Chem. 18 137-158
-
Stryer, L., (1981) Biochemistry, W.H. Freeman & Co., New York pp. 319-320
-
Sweeney, W.V. and Rabinowitz, J.C. (1980) Proteins containing 4Fe-4S clusters:
an overview, Annu. Rev. Biochem. 49, 139-161
-
Voet, D. and Voet, J.G. (1990) Biochemistry, John Wiley & Sons, New
York, pp. 532-544
Last updated 14th April '97
 Click here for a diagram of this model.
Click here for a diagram of this model.